Others
Materials
You can select various silicone rubber materials according to your application.
| Item | General grade |
Medical grade |
Medium-term implant |
Mid-term implant |
|---|---|---|---|---|
| Implantation for 7 days | ー | ● | ● | ● |
| Implantation for 30 days | ー | ー | ● | ● |
| Implantation for 90 days | ー | ー | ー | ● |
| Intracutaneous reactivity(USP) | ー | ー | ○ | ○ |
| Acute systemic toxicity(USP) | ー | ー | ○ | ○ |
| Cytotoxicity | ー | ○ | ○ | ○ |
| Skin sensitization | ー | ー | ○ | ○ |
| Skin irritation | ー | ー | ○ | ○ |
| Weight loss upon heating(EP) | ー | ー | ○ | ○ |
| Pyrogen test(USP) | ー | ー | ー | ○ |
| Hemolysis test | ー | ー | ー | ○ |
| Mutagenicity | ー | ー | ー | ○ |
| Class IV(USP) | ー | ○ | ○ | ○ |
Note: Discussions are required separately for the items marked with ● regarding their use.
Applications
- ● Packings for dialyzers
- ● Caps for syringes
- ● Dishes for cell culture
- ● Check valves for blood circuits and infusion circuits
- ● Components for laparoscopic surgery(trocar)
- ● Various sealing materials for pharmaceuticals, test fluids, etc.
- ● Biological models
- ● Other medical-related diaphragms, O-rings, packings, etc.
Production environment
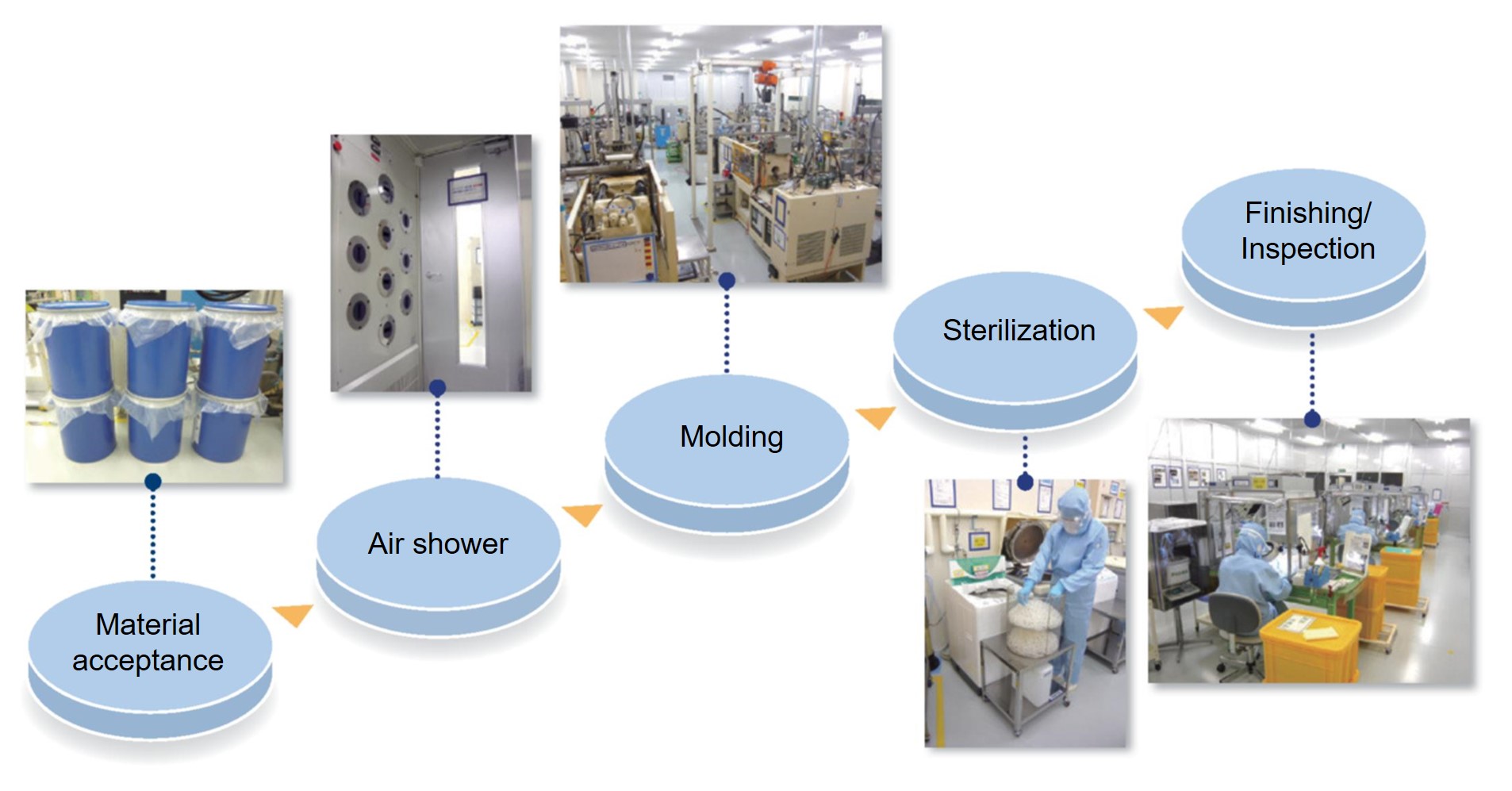
Design and analysis
Design and analysis support optimal product design by enhancing functionality at the design stage, regardless of material, type, or specifications.
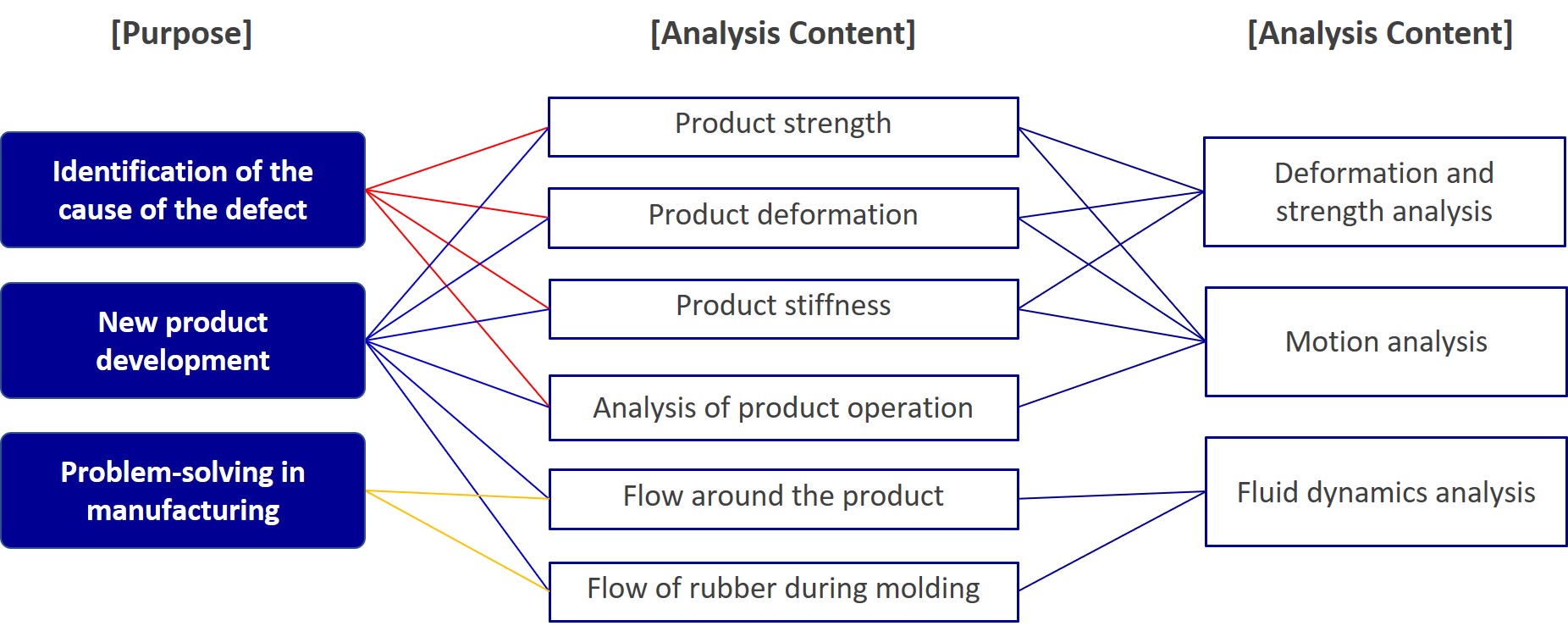
Analysis Examples
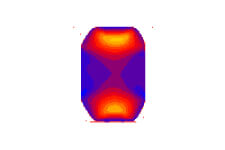 Compression analysis of gaskets
Compression analysis of gaskets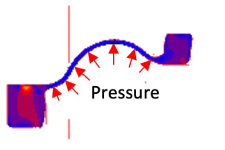 Stress analysis of diaphragms
Stress analysis of diaphragms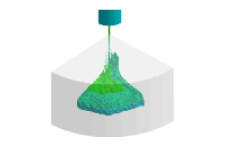 Injection molding analysis of rubber
Injection molding analysis of rubber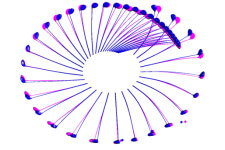 Swing simulation of golf clubs
Swing simulation of golf clubs
ISO13485 Certification
We obtained ISO 13485 certification in October 2018. ISO 13485 is an international standard for quality management systems specific to medical devices.
- Registration date
- October 23 2018
- Registered plant
- Iwatsuki, Haramachi, Headquarters
- Registration number
- ISO13485-0071743
- Certification body
- LRQA




